ELECTRONOMIC ATOMIC THEORY (by Allan Poe Bona Redoña)
Scientific Atoms
Extracting the Electrons out In 1886 Eugen Goldstein after flushing out a tube prior to evacuation for electron radiation’s experiment, he discovered that another ray was moving opposite to electron ray and J.J. Thomson called it ‘positive ray.’ It was discovered that little amount of hydrogen gas in a cathode-ray tube when irradiated can form into electron ray and positive ray. Rutherford called the particles of the positive ray, protons.
This phenomenon happens if electric current is fired through that vacuum tube from cathode to anode terminal causing a pulling out of hydrogen electrons, being dented, from hydrogen proton. The extracted electron is ionized. The electric current’s ionizing (pulling out) energy to extract out electron from hydrogen atom is 13.595 eV.
The same amount of sun’s ray or heat energy should be applied to extract electron from H atom.
Interrelationship
Electron, electric current, and heat energy have an interrelationship.
Magnetism
Facts: When there is an electric current, ther is a magnetic field perpendicular to it.
A ferromagnetic object can be magnetized or demagnetized by certain application of electric current. In the latter capability the heat energy is somewhat similar to electric current.
Heating a magnet up to the Curie point can demagnetize it.
This interrelationship of electric current, heat energy, and electrons can give us hints about the configuration of an atom.
Some of our bases to decipher the Model of Atom
1) It is a fact that pengraletic electron junctions two atoms, as what we can see on the picture of gold atoms.
2) Holding atoms in that manner suggests that both ends (roots) of pengraletic electrons are dented (pierced in) to those atoms.
3) Ionizing electric current, heat radiation, or electromagnetic radiation can extract some electrons from atoms (as light ray can pull out electrons from zinc surface atoms in ring-phenomenon or enough heat energy can split gas molecule).
4) More surpricingly, although has already known long time ago, a movement of strong magnetic field on a coil of a conductor in electric circuit can pull out electrons.
5) Either the conductor’s coil or the magnet should be moved to move (conduction) electrons, so that kinetic energy is needed.
6) Electric current is perpendicular to magnetic field.
7) Electric current can make manifest magnetic field, and magnetic field can make to happen electric current, so that reversal is possible.
Lateral View of Negatively Chraged part of Electro-magnetic induction atom
Top View of an Atom’s Model
7) The amount of voltage (electromotive force) produced by electro-magnetic induction is affected by the speed and amount of the magnetic field and the length of the condcutor in a coil.
Volt, therefore, in an atomic scale is the speeding magnetic field in a distance or the product of the atom’s length (L), atomic magnetic field (B) from another atom above it, and the speed (v) of the transfer of magnetic influence from the said another atom to it.
V = B L v
Volt is directly proportional to the rate of change (alteration) of electron-per-second multiplied by inductance (i.e. a measure of induced voltage by a variation of electron-per-second in the circuit).
Atomic magnetic field serves as a relay to transfer the ‘pushing influence’ to pull out electron, and this ‘relay of transfer of electric influence’ can cause delay or resistance. We can know the electric resistance (ohm) by dividing the produced electron-per-second or electric charge from BLv or volt. Electric resistance is the electromotive force (volt) expended per produced electron-per-second.
We can summarize it as: the closeness or the maximum distance of conductiong atoms to each other in series can cause electric resistance (ohm).
Therefore, no volt (electromotive force) is required to pull out conduction-electrons if the conducting atoms are attached body to body. For pure metallic element this body (atom) to body (atom) bonding can be achieved by much stronger arepellic (cold) field.
It is a fact that electric resistance (Ω) increases in linear direction (or perhaps in capacitoric-pengraletic direction) and grows weaker in voluminous direction (or apparently in concentrical direction).
A series conducting atroms has a higher resistance (Ω), whereas a parallel of series conducting atoms in a length has a lower electric resistance.
It is tempting to think that the deeper the inductoric (concentrical) layer in the atom, the higher the electric resistance, whereas the upper the capacitoric ßeta photons, the stronger the ohm (Ω).
9) As the magnetic field huffs (sinks) on the conductor’s atoms, the atoms will puff (exhale) their electrons if vacuum exist between those cathodic atoms and anode.
In reversed condition, if electrons are puffed (pulled out), negative charge will exist and magnetic field will be extracting from (huffings in) the atoms. It is a matter of action (pushing electron) and reaction 9 pulling magnetic filed).
The absence of electron which causes a magnetic pulling is what we called Redoña condition.
Redoña condition exists in the atom as either there is an occurrence of a pulling (puffing) out of electrons or intrusion (huffing) of magnetic field.
A series od Redoña condition is what we called current electricity with ohm (Ω).
The quantity (n) of magnetically projected electron per atom is calculable by
where
is the inductoric field (atomic magnetic field, in tesla) speeding (v) across the conductor’s atom, π is the constant pi, r is the radius (in meter) of the conductor’s atom (including the pengraletic bond length at one side) at room temperature, p is the electrical resistivity (in ohm meter) of the conductor, and
is the electron’s electric charge constant per second (or 0.000 000 000 000 000 000 160 217 487 coulomb per second).
The atom which projects those conduction electrons has an electric field ( Fe) determinable by
Fe = Q p / t π r ²
where Q is the total electric charge of those conduction electrons in time (t) of one second.
Electric field (which is measured in volt per meter) is counteracted by or equivalent to a speeding magnetic field, exerting a force per electric charge.
Magnetic field cannot affect electric field unless it has received kinetic energy, and vice versa.
The kinetic energy (Ke) required to exert magnetic atomic (inductoric) field is determinable by
where m is the atom’s mass (in kilogram), Q is the electric charge (in coulomb) of the pulled out (projected) electrons in time (t, in second), p is the conductor’s electric resistivity, and r is the radius of the conductor’s atom (included the length of the atom’s pengralet at one side).
Stable atom of a conductor has been utilizing no magnetic kinetic energy when there is no Redoña condition (external disturbance of electric or magnetic field) to the atom.
On contrary, it is apparent that unstable atom of radioactive element is continuously utilizing such kinetic energy even in the absence of foreign disturbances (electric and/or magnetic).
It is a leaking potential energy. A stable particle has a potential energy if at rest and a kinetic energy if moving. On the other hand, unstable particle (e.g. radioactive substance) has a leakage of potential energy even not moving. This leakage tends the particle to move but it cannot, so if it is in capable enough it will eject rather its cause (usually ßeta photons, alpha photons, or chadwick ßetons), changing the volume or charge of the particle.
The leaking potential energy ( P e ) is possibly calculable by the equation
Pe = ½ [1 - (m/M) ² ] Em ,
where m is the new stable mass, M is the former unstable mass, and Em is the total energy-mass (or the absolute content, in joule) of the unstable mass.
To counteract this leakage (i.e. utilizing kinetic energy without moving), the unstable mass must be moved with a kinetic energy equivalent to the said potential energy. However, since that potential energy cannot eject the unstable mass, it will rather emit the much lesser weighing atomic ‘infectious’ (or isntability-causing) photons (in a form of ßetons, alpha photons, gluonic ßetons, or gamma photons or combination, depending on the amount). What will be left to the mass is the stability potential energy for rutherford (nucleons). However, still slight instability exists but this time to the valence electrons.
Nobel gases (helium, neon, argon, etc.), were once called inert because they, have most stable valence electrons, whereas fluorine and oxygen have least stable valence electrons in terms of valential condition.
Oxygen molecule’s atom ‘feels’ deficient (of two electrons) when enough kinetic energy from foreign molecules ‘knocks’ on it. Fluorine, however, ‘feels’ a deficiency of one electron if “knocks’ by drawing near foreign molecule. To fill their valential instability in they are partly pulling out the valence electrons of other atoms and insert to their bodies. On contrary, sodium and magnesium have weaker dented valence electrons, and have a tendency to partly give up their weaker valence electrons. In this scenario, their valence electrons are atomic ’sex organs’ - the inserting ones being ‘male’ and the oxygen or fluorine being ‘female’. Hydrogen -like perhaps all other elements- is hermaproditic, although more oftenly having a ‘male’ trait. this is the basic unit of sexual activity in the nature, leading to a more complicated organismic sexual activities.
How about magnetically pulling those electrons out halfway (without completely extracting them) from their atoms with Redoña condition?
This is what happening to a paramagnetic substance.
In the case of ferromagnets (iron, nickel, or cobalt), the Redoña conditional electrons remain in more than half-way condition even the electricity or foreign magnetic field is gone. As those electrons remain in that suspended condition, huffing (extracting in) balanons -magnetic photons- exist perpendicular to their direction.
Seductom
The effect of Redoña condition to the atoms is the making them seductomic.
What is seductom?
Seductom is a type of atom which () is foreign-magnetically oriented due to its Redoña condition susceptibility and (2) can receive or pass electricity by atomic magnetic (inductoric) field, instead of direct electric (ßetons) to electric (ßetons). Indirectly the length of pengraletic bond causes atoms to become seductomic. Seductoms become conductoms if they (atoms) have pierced in each one another.
Lead (Pb), for an instance, has no seductom and consisted of conductoms at the arepellic (cold) field 7.2 kelvin (Celsius) above absolute zero, whereas platinum has none seductom at 0.0019 kelvin or lithium at 0.0004 kelvin. The electricity at this condition can transfer influence (”current”) by only knocking the atoms, and those atoms need not to relay the influence by atomic magnetic fields but directly by ßeta photons of the atoms, as a result no electromotive force (volt) is required to push conduction electrons and, in effect, no electric resistance (Ω).
The increase of conduction atom’s pengraletic length is the increase of seductom.
The quantity of pulled out electron (ßo) per second is directly proportional to the number of priductom or of ‘transfering’ atoms (n) and inversely proportional to the number of seductom (ns) and then multiply by the number of electricity’s knocking electron (ßi) per second.
For example, if one seductom is receiving atomic magnetic influence to 1 million priductoms (primary induction atoms), the conducting (pulled out) electrons is 2 000 000 per second, if 2 electrons per second have knocked those priductoms.
2 ß/s x (1000000 priductoms/1 seductom) = 2000000 pulled out electrons per second
The voltage expended to move those 2 million ß per second is 0.000 002 volt.
A conductor without seductom is a superconductor, whose atoms is having atomic magnetic alignment 90 degrees at certain distance to one another. Temperature and pressure can affect this superconductive (zero seductom) condition of a conductor.
For ceramic and other mixtures of various elements superconductive condition can be reached even at certain higher tenperatures. But for pure conductive elements it can be achieved at much lower temperature, usually nearer to Absolute Zero. This suggests that a modified version of ball-and-stick model is the appropriate model for pure gaseous elements at higher temperature.
The atoms of pure element at higher temperature are not in this superconductive alignment but distant apart by pengraletic junctions due to atomic repulsion caused by disturbing heat ray from the surrounding.
The most important rediscovery we have particular with this paper is that when strong foreign magnetic field moved across the electromagnet (coil of an electric circuit), the influence will be passed as possible as to the cathode terminal (from which radiation or ionic electrons can jump out).
We can imagine electron as root (capacitoric) and tooth (pengraletic) and its dent (puncture) in the atom is the heisenberg passage; and the atom is as a spherical gum.
Electric current, heat energy, and radiation as drills can loosen the capture of gum (atom) to the teeth (pengraletic electrons) and, if enough energy is applied, can even extract or puff those teeth (electrons) from the gum (atom).
Some gums (atoms) are bleeding; those are the radioactive elements and they bleed because of the atomic rutherford ‘infections’ (excess ßeta and/or alpha photons) .
Other gums (atoms) don’t have ‘porcelain’ teeth but ‘rubber teeth,’ whereas others have ‘jelly teeth.’ Porcelain teeth are interlocking pengraletic electrons, rubber teeth are conjoined pengralets, whereas jelly teeth are conjoined tribons. Atoms bite another atoms to form an object. Thus, an object with ‘porcelain teeth’ is solid, whereas with rubber teeth is elastic, soft solid, and those with jelly teeth are liquids, and those with a combination of rubber & jelly teeth are pasty.
Pulling teeth (electrons) out will cause pain in the gum, and that ‘pain’ is what we called magnetic field. Now, if the teeth (electrons) are halfway pulled yet still their roots & nerves are magnetically connected to the gum (atom), the tootache (magnetic field) will remain as what can happen to ferromagnets. Thus, iron magnet mean it has achinh gums (atoms). However, almost all conductors have anesthetic gums (atoms) that’s why tootache will go away in the absence of current electricity.
The ionization energy needed to pull out a tooth (electron) varies depending on the deepness (and priority) of the tooth’s root in the gum. The longest root is the deepest pierced in capacitoric electron needs much higher ionization energy to be extracted. The root (capacitoric) of the tooth (electron) is connected to a group of nerves (chadwick ßetons). Chadwick ßetons are hydrogen complex -made up of gluonically interlocked proton, electron & neutrino, and well known as neutron. above and below this layer (neutron) are the layers of alpha photons 9i.e. protons). Alpha-photons are gluonic-beta photons.
When tooth (electron) is fully pulled out, the gum (atom) will shrink. thus, the more teeth (electrons) are extracted from it, the more shrank the gum (atom) has.
Denting extra tooth (electron) without adding nerves (chadwick ßeta photons) and flesh (alpha photons) will cause an inflamation to the gum (atom). And yet adding extra layer of chadwick ßetons (neutron) and layer of alpha-photons (proton) requires enough energy and the gum (atom), during the process, will shrink drastically due to strong atomic repulsion caused by the adding nucleons & energy. The shrank atom in this condition is what we called nuclear rutherford (concentrical layers of nucleons). The rutherford has a tendency to divide into two, or to form nuclear molecule (that is, molecule of nucleons).
Neutron is the particle of neutron radiation and it is believed to be the chadwick ßetons outside the atom.
Proton is alpha-photons outside the atom, or the particle of positive radiation.
The Redoña Frame is supposed to be, or at least approximates, the atomic genetic framework. It can predict many things about the atoms and elements some of which are the spiral inductoric layers during Redoña condition or apparently the involutional concentric configuration of pauli layers, the oxidation susceptibility of krypton (Kr), xenon (Xe) and radon (Rn), the existence of VIII transition group (those in rectangles), the existence of main levels or groupings of pauli layers, the periodic peak of electronegativity (circle) among the elements, the posibility of feminine valence race (example are those inlips), masculine valence or hermaproditic valnce, the periodic peak of the 1st ionization energy (left corner of the spiral), the location of liquid metals, metalloids and gases in the periodic table; and it may suggest why magnetic field can be polarized by electromagnet’s coil (spiral), and cam give us reason that as a template can partly cause electron radiation to spiral gyrate in strong magnetic field, and show us why elements are not smoothly accendingly arranged in the periodic table.
It also predicts the sideway semiliraties between titanium (Ti), zirconium (Zr), haftnium (Hf), and germanium (Ge), tin (Sn), & lead (Pb). Vertical similarities between lithium (Li), beryllium (Be), and boron (B) to magnesium (Mg), aluminum (Al), and silicon (Si) are also possible.
All isotopes outside the spiral of stable zone are unstable.
The open and uppermost portion of the frame or spiral is the unstable zone which starts two steps backward before the radon to throughout the siegbahn layer. All isotopes in this zone are radioactive and thought to be have no stable form.
Anlagyurtal (Center of the Atom)
Neuron is the particle of neutron radiation outside the atom.
In the atom it is the chadwick gluonic ßeta photons.
Redoña Frame and simuoval function are probably some activities of the atom’s center.
Originally, this neutron is a hydrogen complex made up of supposed to be compressed proton, electron, antineutrino & energy. Theoretically the atomic center is made up of this chadwick hydrogen which we called anlagyurtal.
The anlagyurtal nucleon in the center is theoretically a genetic compass, for it supposed to be influencing the direction of dentation of the electrons and apparently dictate the functions of an atom of a particular mass.
The diameter of anlagyurtal nucleon is constant to a particular ionization energy. The greater the ionization energy knocking on the atom disturbing the anlagyurtal, the smaller the anlagyurtal’s diameter. This suggests that ionizing energy can pull out electrons, disturbing the atom and affecting the diameter of the anlagyurtal.
To determine the reduced anlagyurtal diameter (Liß) caused by an ionizing energy (E), the formula is
L i β = И / E
where И is the product of K e ² (where e is the electron’s electric charge constant, and K is the MKS applied in Coulomb’s Law equivalent to 1/4π ε0, where ε 0 is the free-space electric constant). The value of И is approximately 2.307773 x 10 - 28 joule meter. If the ionizing energy or projectile fired on it is equivalent to the energy-mass of an electron radiation, the L i β has a value of Ro or Comptom scattering effect.
The anlagyurtal nucleon reaches the twice Bohr constant (i.e. hydrogen diameter) if disturbed by an ionizing energy equivalent to the 1st ionization energy of the hydrogen atom.
We can calculate the anlagyurtal diameter (d) per electronvolt of ionizing energy ( J ) by
d = 1. 439 964 6 x 10 -9 electronvolt meter / J .
The anlagyurtal nucleon targetter by an electron radiation reduces in diameter to more or less, presumably, one fermi or O.000 000 000 000 001 meter (or 1 femtometer) called atomic nucleus, and has a tendency to clump into two.
Images Credits:
photograph of gold atoms - Science Photo Library, Physics Today The World Book Encyclopedia of Science, page 15. Verlagsgruppe Bertelsmann International GmbH, Munich 1984, published by World Book, Inc., Chicago, revised edition 1987.
drawings of seductom, atomic model, and conductoms - Allan Poe Bona Redoña
Read also:
Click here ► What is Gravity ?
►Facts about Atoms
►What is Magnetism ?
Extracting the Electrons out In 1886 Eugen Goldstein after flushing out a tube prior to evacuation for electron radiation’s experiment, he discovered that another ray was moving opposite to electron ray and J.J. Thomson called it ‘positive ray.’ It was discovered that little amount of hydrogen gas in a cathode-ray tube when irradiated can form into electron ray and positive ray. Rutherford called the particles of the positive ray, protons.
This phenomenon happens if electric current is fired through that vacuum tube from cathode to anode terminal causing a pulling out of hydrogen electrons, being dented, from hydrogen proton. The extracted electron is ionized. The electric current’s ionizing (pulling out) energy to extract out electron from hydrogen atom is 13.595 eV.
The same amount of sun’s ray or heat energy should be applied to extract electron from H atom.
Interrelationship
Electron, electric current, and heat energy have an interrelationship.
Magnetism
Facts: When there is an electric current, ther is a magnetic field perpendicular to it.
A ferromagnetic object can be magnetized or demagnetized by certain application of electric current. In the latter capability the heat energy is somewhat similar to electric current.
Heating a magnet up to the Curie point can demagnetize it.
This interrelationship of electric current, heat energy, and electrons can give us hints about the configuration of an atom.
Some of our bases to decipher the Model of Atom
1) It is a fact that pengraletic electron junctions two atoms, as what we can see on the picture of gold atoms.
2) Holding atoms in that manner suggests that both ends (roots) of pengraletic electrons are dented (pierced in) to those atoms.
3) Ionizing electric current, heat radiation, or electromagnetic radiation can extract some electrons from atoms (as light ray can pull out electrons from zinc surface atoms in ring-phenomenon or enough heat energy can split gas molecule).
4) More surpricingly, although has already known long time ago, a movement of strong magnetic field on a coil of a conductor in electric circuit can pull out electrons.
5) Either the conductor’s coil or the magnet should be moved to move (conduction) electrons, so that kinetic energy is needed.
6) Electric current is perpendicular to magnetic field.
7) Electric current can make manifest magnetic field, and magnetic field can make to happen electric current, so that reversal is possible.
negative part of electricity
Top View of an Atom’s Model
7) The amount of voltage (electromotive force) produced by electro-magnetic induction is affected by the speed and amount of the magnetic field and the length of the condcutor in a coil.
Volt, therefore, in an atomic scale is the speeding magnetic field in a distance or the product of the atom’s length (L), atomic magnetic field (B) from another atom above it, and the speed (v) of the transfer of magnetic influence from the said another atom to it.
V = B L v
Volt is directly proportional to the rate of change (alteration) of electron-per-second multiplied by inductance (i.e. a measure of induced voltage by a variation of electron-per-second in the circuit).
Atomic magnetic field serves as a relay to transfer the ‘pushing influence’ to pull out electron, and this ‘relay of transfer of electric influence’ can cause delay or resistance. We can know the electric resistance (ohm) by dividing the produced electron-per-second or electric charge from BLv or volt. Electric resistance is the electromotive force (volt) expended per produced electron-per-second.
We can summarize it as: the closeness or the maximum distance of conductiong atoms to each other in series can cause electric resistance (ohm).
Therefore, no volt (electromotive force) is required to pull out conduction-electrons if the conducting atoms are attached body to body. For pure metallic element this body (atom) to body (atom) bonding can be achieved by much stronger arepellic (cold) field.
It is a fact that electric resistance (Ω) increases in linear direction (or perhaps in capacitoric-pengraletic direction) and grows weaker in voluminous direction (or apparently in concentrical direction).
A series conducting atroms has a higher resistance (Ω), whereas a parallel of series conducting atoms in a length has a lower electric resistance.
It is tempting to think that the deeper the inductoric (concentrical) layer in the atom, the higher the electric resistance, whereas the upper the capacitoric ßeta photons, the stronger the ohm (Ω).
9) As the magnetic field huffs (sinks) on the conductor’s atoms, the atoms will puff (exhale) their electrons if vacuum exist between those cathodic atoms and anode.
In reversed condition, if electrons are puffed (pulled out), negative charge will exist and magnetic field will be extracting from (huffings in) the atoms. It is a matter of action (pushing electron) and reaction 9 pulling magnetic filed).
The absence of electron which causes a magnetic pulling is what we called Redoña condition.
Redoña condition exists in the atom as either there is an occurrence of a pulling (puffing) out of electrons or intrusion (huffing) of magnetic field.
A series od Redoña condition is what we called current electricity with ohm (Ω).
The quantity (n) of magnetically projected electron per atom is calculable by
where
is the inductoric field (atomic magnetic field, in tesla) speeding (v) across the conductor’s atom, π is the constant pi, r is the radius (in meter) of the conductor’s atom (including the pengraletic bond length at one side) at room temperature, p is the electrical resistivity (in ohm meter) of the conductor, and
is the electron’s electric charge constant per second (or 0.000 000 000 000 000 000 160 217 487 coulomb per second).
The atom which projects those conduction electrons has an electric field ( Fe) determinable by
Fe = Q p / t π r ²
where Q is the total electric charge of those conduction electrons in time (t) of one second.
Electric field (which is measured in volt per meter) is counteracted by or equivalent to a speeding magnetic field, exerting a force per electric charge.
Magnetic field cannot affect electric field unless it has received kinetic energy, and vice versa.
The kinetic energy (Ke) required to exert magnetic atomic (inductoric) field is determinable by
where m is the atom’s mass (in kilogram), Q is the electric charge (in coulomb) of the pulled out (projected) electrons in time (t, in second), p is the conductor’s electric resistivity, and r is the radius of the conductor’s atom (included the length of the atom’s pengralet at one side).
Stable atom of a conductor has been utilizing no magnetic kinetic energy when there is no Redoña condition (external disturbance of electric or magnetic field) to the atom.
On contrary, it is apparent that unstable atom of radioactive element is continuously utilizing such kinetic energy even in the absence of foreign disturbances (electric and/or magnetic).
It is a leaking potential energy. A stable particle has a potential energy if at rest and a kinetic energy if moving. On the other hand, unstable particle (e.g. radioactive substance) has a leakage of potential energy even not moving. This leakage tends the particle to move but it cannot, so if it is in capable enough it will eject rather its cause (usually ßeta photons, alpha photons, or chadwick ßetons), changing the volume or charge of the particle.
The leaking potential energy ( P e ) is possibly calculable by the equation
Pe = ½ [1 - (m/M) ² ] Em ,
where m is the new stable mass, M is the former unstable mass, and Em is the total energy-mass (or the absolute content, in joule) of the unstable mass.
To counteract this leakage (i.e. utilizing kinetic energy without moving), the unstable mass must be moved with a kinetic energy equivalent to the said potential energy. However, since that potential energy cannot eject the unstable mass, it will rather emit the much lesser weighing atomic ‘infectious’ (or isntability-causing) photons (in a form of ßetons, alpha photons, gluonic ßetons, or gamma photons or combination, depending on the amount). What will be left to the mass is the stability potential energy for rutherford (nucleons). However, still slight instability exists but this time to the valence electrons.
Nobel gases (helium, neon, argon, etc.), were once called inert because they, have most stable valence electrons, whereas fluorine and oxygen have least stable valence electrons in terms of valential condition.
Oxygen molecule’s atom ‘feels’ deficient (of two electrons) when enough kinetic energy from foreign molecules ‘knocks’ on it. Fluorine, however, ‘feels’ a deficiency of one electron if “knocks’ by drawing near foreign molecule. To fill their valential instability in they are partly pulling out the valence electrons of other atoms and insert to their bodies. On contrary, sodium and magnesium have weaker dented valence electrons, and have a tendency to partly give up their weaker valence electrons. In this scenario, their valence electrons are atomic ’sex organs’ - the inserting ones being ‘male’ and the oxygen or fluorine being ‘female’. Hydrogen -like perhaps all other elements- is hermaproditic, although more oftenly having a ‘male’ trait. this is the basic unit of sexual activity in the nature, leading to a more complicated organismic sexual activities.
How about magnetically pulling those electrons out halfway (without completely extracting them) from their atoms with Redoña condition?
This is what happening to a paramagnetic substance.
In the case of ferromagnets (iron, nickel, or cobalt), the Redoña conditional electrons remain in more than half-way condition even the electricity or foreign magnetic field is gone. As those electrons remain in that suspended condition, huffing (extracting in) balanons -magnetic photons- exist perpendicular to their direction.
Seductom
The effect of Redoña condition to the atoms is the making them seductomic.
What is seductom?
Seductom is a type of atom which () is foreign-magnetically oriented due to its Redoña condition susceptibility and (2) can receive or pass electricity by atomic magnetic (inductoric) field, instead of direct electric (ßetons) to electric (ßetons). Indirectly the length of pengraletic bond causes atoms to become seductomic. Seductoms become conductoms if they (atoms) have pierced in each one another.
Lead (Pb), for an instance, has no seductom and consisted of conductoms at the arepellic (cold) field 7.2 kelvin (Celsius) above absolute zero, whereas platinum has none seductom at 0.0019 kelvin or lithium at 0.0004 kelvin. The electricity at this condition can transfer influence (”current”) by only knocking the atoms, and those atoms need not to relay the influence by atomic magnetic fields but directly by ßeta photons of the atoms, as a result no electromotive force (volt) is required to push conduction electrons and, in effect, no electric resistance (Ω).
The increase of conduction atom’s pengraletic length is the increase of seductom.
The quantity of pulled out electron (ßo) per second is directly proportional to the number of priductom or of ‘transfering’ atoms (n) and inversely proportional to the number of seductom (ns) and then multiply by the number of electricity’s knocking electron (ßi) per second.
For example, if one seductom is receiving atomic magnetic influence to 1 million priductoms (primary induction atoms), the conducting (pulled out) electrons is 2 000 000 per second, if 2 electrons per second have knocked those priductoms.
2 ß/s x (1000000 priductoms/1 seductom) = 2000000 pulled out electrons per second
The voltage expended to move those 2 million ß per second is 0.000 002 volt.
A conductor without seductom is a superconductor, whose atoms is having atomic magnetic alignment 90 degrees at certain distance to one another. Temperature and pressure can affect this superconductive (zero seductom) condition of a conductor.
For ceramic and other mixtures of various elements superconductive condition can be reached even at certain higher tenperatures. But for pure conductive elements it can be achieved at much lower temperature, usually nearer to Absolute Zero. This suggests that a modified version of ball-and-stick model is the appropriate model for pure gaseous elements at higher temperature.
The atoms of pure element at higher temperature are not in this superconductive alignment but distant apart by pengraletic junctions due to atomic repulsion caused by disturbing heat ray from the surrounding.
The most important rediscovery we have particular with this paper is that when strong foreign magnetic field moved across the electromagnet (coil of an electric circuit), the influence will be passed as possible as to the cathode terminal (from which radiation or ionic electrons can jump out).
We can imagine electron as root (capacitoric) and tooth (pengraletic) and its dent (puncture) in the atom is the heisenberg passage; and the atom is as a spherical gum.
Electric current, heat energy, and radiation as drills can loosen the capture of gum (atom) to the teeth (pengraletic electrons) and, if enough energy is applied, can even extract or puff those teeth (electrons) from the gum (atom).
Some gums (atoms) are bleeding; those are the radioactive elements and they bleed because of the atomic rutherford ‘infections’ (excess ßeta and/or alpha photons) .
Other gums (atoms) don’t have ‘porcelain’ teeth but ‘rubber teeth,’ whereas others have ‘jelly teeth.’ Porcelain teeth are interlocking pengraletic electrons, rubber teeth are conjoined pengralets, whereas jelly teeth are conjoined tribons. Atoms bite another atoms to form an object. Thus, an object with ‘porcelain teeth’ is solid, whereas with rubber teeth is elastic, soft solid, and those with jelly teeth are liquids, and those with a combination of rubber & jelly teeth are pasty.
Pulling teeth (electrons) out will cause pain in the gum, and that ‘pain’ is what we called magnetic field. Now, if the teeth (electrons) are halfway pulled yet still their roots & nerves are magnetically connected to the gum (atom), the tootache (magnetic field) will remain as what can happen to ferromagnets. Thus, iron magnet mean it has achinh gums (atoms). However, almost all conductors have anesthetic gums (atoms) that’s why tootache will go away in the absence of current electricity.
The ionization energy needed to pull out a tooth (electron) varies depending on the deepness (and priority) of the tooth’s root in the gum. The longest root is the deepest pierced in capacitoric electron needs much higher ionization energy to be extracted. The root (capacitoric) of the tooth (electron) is connected to a group of nerves (chadwick ßetons). Chadwick ßetons are hydrogen complex -made up of gluonically interlocked proton, electron & neutrino, and well known as neutron. above and below this layer (neutron) are the layers of alpha photons 9i.e. protons). Alpha-photons are gluonic-beta photons.
When tooth (electron) is fully pulled out, the gum (atom) will shrink. thus, the more teeth (electrons) are extracted from it, the more shrank the gum (atom) has.
Denting extra tooth (electron) without adding nerves (chadwick ßeta photons) and flesh (alpha photons) will cause an inflamation to the gum (atom). And yet adding extra layer of chadwick ßetons (neutron) and layer of alpha-photons (proton) requires enough energy and the gum (atom), during the process, will shrink drastically due to strong atomic repulsion caused by the adding nucleons & energy. The shrank atom in this condition is what we called nuclear rutherford (concentrical layers of nucleons). The rutherford has a tendency to divide into two, or to form nuclear molecule (that is, molecule of nucleons).
Neutron is the particle of neutron radiation and it is believed to be the chadwick ßetons outside the atom.
Proton is alpha-photons outside the atom, or the particle of positive radiation.
The Redoña Frame is supposed to be, or at least approximates, the atomic genetic framework. It can predict many things about the atoms and elements some of which are the spiral inductoric layers during Redoña condition or apparently the involutional concentric configuration of pauli layers, the oxidation susceptibility of krypton (Kr), xenon (Xe) and radon (Rn), the existence of VIII transition group (those in rectangles), the existence of main levels or groupings of pauli layers, the periodic peak of electronegativity (circle) among the elements, the posibility of feminine valence race (example are those inlips), masculine valence or hermaproditic valnce, the periodic peak of the 1st ionization energy (left corner of the spiral), the location of liquid metals, metalloids and gases in the periodic table; and it may suggest why magnetic field can be polarized by electromagnet’s coil (spiral), and cam give us reason that as a template can partly cause electron radiation to spiral gyrate in strong magnetic field, and show us why elements are not smoothly accendingly arranged in the periodic table.
It also predicts the sideway semiliraties between titanium (Ti), zirconium (Zr), haftnium (Hf), and germanium (Ge), tin (Sn), & lead (Pb). Vertical similarities between lithium (Li), beryllium (Be), and boron (B) to magnesium (Mg), aluminum (Al), and silicon (Si) are also possible.
All isotopes outside the spiral of stable zone are unstable.
The open and uppermost portion of the frame or spiral is the unstable zone which starts two steps backward before the radon to throughout the siegbahn layer. All isotopes in this zone are radioactive and thought to be have no stable form.
Anlagyurtal (Center of the Atom)
Neuron is the particle of neutron radiation outside the atom.
In the atom it is the chadwick gluonic ßeta photons.
Redoña Frame and simuoval function are probably some activities of the atom’s center.
Originally, this neutron is a hydrogen complex made up of supposed to be compressed proton, electron, antineutrino & energy. Theoretically the atomic center is made up of this chadwick hydrogen which we called anlagyurtal.
The anlagyurtal nucleon in the center is theoretically a genetic compass, for it supposed to be influencing the direction of dentation of the electrons and apparently dictate the functions of an atom of a particular mass.
The diameter of anlagyurtal nucleon is constant to a particular ionization energy. The greater the ionization energy knocking on the atom disturbing the anlagyurtal, the smaller the anlagyurtal’s diameter. This suggests that ionizing energy can pull out electrons, disturbing the atom and affecting the diameter of the anlagyurtal.
To determine the reduced anlagyurtal diameter (Liß) caused by an ionizing energy (E), the formula is
L i β = И / E
where И is the product of K e ² (where e is the electron’s electric charge constant, and K is the MKS applied in Coulomb’s Law equivalent to 1/4π ε0, where ε 0 is the free-space electric constant). The value of И is approximately 2.307773 x 10 - 28 joule meter. If the ionizing energy or projectile fired on it is equivalent to the energy-mass of an electron radiation, the L i β has a value of Ro or Comptom scattering effect.
The anlagyurtal nucleon reaches the twice Bohr constant (i.e. hydrogen diameter) if disturbed by an ionizing energy equivalent to the 1st ionization energy of the hydrogen atom.
We can calculate the anlagyurtal diameter (d) per electronvolt of ionizing energy ( J ) by
d = 1. 439 964 6 x 10 -9 electronvolt meter / J .
The anlagyurtal nucleon targetter by an electron radiation reduces in diameter to more or less, presumably, one fermi or O.000 000 000 000 001 meter (or 1 femtometer) called atomic nucleus, and has a tendency to clump into two.
Images Credits:
photograph of gold atoms - Science Photo Library, Physics Today The World Book Encyclopedia of Science, page 15. Verlagsgruppe Bertelsmann International GmbH, Munich 1984, published by World Book, Inc., Chicago, revised edition 1987.
drawings of seductom, atomic model, and conductoms - Allan Poe Bona Redoña
Read also:
Click here ► What is Gravity ?
►Facts about Atoms
►What is Magnetism ?






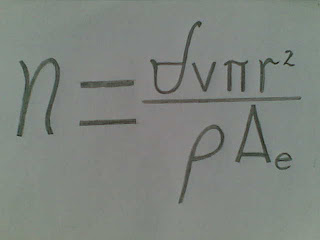










No comments:
Post a Comment